Muhammad Imran, PhD
Tagline:Research Scientist, Synthesis of Molecular Quantum Materials
Dresden, Germany

About Me
I am a synthetic organic chemist with expertise in multi-step synthesis of organic molecular quantum materials. I am particularly interested in unique physical properties of molecular quantum materials for future quantum technologies including quantum computing, quantum sensing and quantum communications.
Education & Training
Research Interests
- Organic Synthesis
- Materials Chemistry
- Quantum Information Science
Honors & Awards
Publications
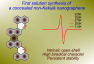
A Persistent Concealed Non-Kekulé Nanographene: Synthesis and in-situ Characterization
Journal ArticlePublisher:Organic Chemistry FrontiersDate:2025Authors:Muhammad ImranLin YangJinjiang ZhangYubin FuZhen-Lin QiuNoel IsraelEvgenia DmitrievaGianluca SerraAndrea LucottiMatteo TommasiniJi MaXinliang FengDescription:Concealed non-Kekulé (CNK) nanographenes have recently gained attention as promising non-Kekulé model systems due to their distinctive antiferromagnetic electronic spins, which offer potential applications in spintronics and quantum information science. However, synthesizing CNK nanographenes in solution remains a significant challenge because of their strong biradical character and high reactivity. In this study, we report the successful synthesis of a novel CNK nanographene with two phenalene units fused in a cis configuration to perylene (c-CNK), which exhibits persistent stability under ambient conditions, with a half-life (t1/2) of 59 minutes. The formation of the c-CNK is confirmed using in-situ UV-Vis-NIR spectroscopy, Raman spectroscopy, and high-resolution mass spectrometry. The open-shell character of c-CNK is supported by the electron paramagnetic resonance (EPR) spectroscopy by observing an isotropic signal with a g-value of 2.0026. Quantum chemical simulations reveal a high biradical character (y0 = 0.97) and a singlet open-shell ground state with a small singlet-triplet energy gap (ΔES-T) of 0.4 kcal/mol. This work presents a solution synthesis of a next-generation concealed non-Kekulé nanographene with intrinsic antiferromagnetic electronic spins, highlighting its potential as promising material for future quantum technologies.
Chemically Triggered Release of Singlet Oxygen from Bisphenalenyl Endoperoxides with a Brønsted Acid
Journal ArticlePublisher:Organic LettersDate:2022Authors:Muhammad ImranMark S. ChenDescription:Aromatic endoperoxides have emerged as intriguing stimulus-responsive materials for molecular oxygen (O2) storage and delivery but are currently limited in their application because they require heat to trigger O2 release. Here we present the first example of acid-triggered singlet oxygen (1O2) release that does not require external heating by treating bisphenalenyl endoperoxides (EPOs) with trifluoroacetic acid. Mechanistic studies reveal that diprotonation of EPOs leads to a >10-fold increase in cycloreversion rates by lowering the energy of activation (ΔEa) by as much as 71.1 kJ mol–1. Remarkably, acid-catalyzed 1O2 release is even demonstrated at room temperature. Chemical trapping experiments indicate that reactive 1O2 is present during acid-triggered release, which is promising for the development of these molecular materials for metal-free, on-demand 1O2 delivery.
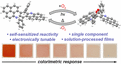
Self-Sensitized and Reversible O2 Reactivity with Bisphenalenyls for Simple, Tunable, and Multicycle Colorimetric Oxygen-Sensing Films
Journal ArticlePublisher:ACS Applied Materials & InterfacesDate:2021Authors:Muhammad ImranMark S. ChenDescription:Monitoring the levels of molecular oxygen (O2) is critical for numerous applications, but there is still a long-standing challenge to develop robust and cost-effective colorimetric sensors that enable detection by changes in color. Current technologies employ chromophores that require additional additives, which inherently increase the cost and complexity. Here, we report that bisphenalenyls (PQPLs) function as the single active materials for colorimetric O2 sensing through their quantitative conversion into aromatic endoperoxides (EPOs). PQPLs display self-sensitizing reactivity: they are capable of generating singlet oxygen and binding it without the need for external photosensitizers. The rates of PQPL photooxygenation depend on the electron-donating ability of substituents, which highlights a simple strategy for tuning O2 sensitivity. EPOs are stable under ambient conditions but can be thermally stimulated to convert back to PQPLs and concomitantly release O2. Polymer-supported (PTMSP) films of PQPLs (2 wt %) reproduce these reactivity trends with a rapid red-to-colorless transition that is visible to the naked eye within 1 h of exposure and show a very low limit of detection (<5 ppm O2). Films are chemically and thermally robust and maintain up to >99% of their original colorimetric response when reused and subjected to multiple cycles of photooxygenation and O2 release. The simplicity and solution processability of these materials highlight their potential as “intelligent” inks for printable colorimetric sensors.
Spin multiplicity effects in doublet versus singlet emission: the photophysical consequences of a single electron
Journal ArticlePublisher:Chemical ScienceDate:2020Authors:Caleb M. WehrmannMuhammad ImranCraig PointerLisa A. FredinElizabeth R. YoungMark S. ChenDescription:Ambient-stable fluorescent radicals have recently emerged as promising luminescent materials; however, tailoring their properties has been difficult due to the limited photophysical understanding of open-shell organic systems. Here we report the experimental and computational analysis of a redox pair of π-conjugated fluorescent molecules that differ by one electron. A π-dication (DC) and π-radical cation (RC) demonstrate different absorption spectra, but similar red emission (λemiss,max = ∼630 nm), excitation maxima (λexc,max = ∼530 nm), fluorescence lifetimes (1–10 ns), and even excited-state (non-emissive) lifetimes when measured by transient absorption spectroscopy. Despite their experimental similarities, time-dependent density functional theory (TDDFT) studies reveal that DC and RC emission mechanisms are distinct and rely on different electronic transitions. Excited-state reorganization occurs by hole relaxation in singlet DC, while doublet RC undergoes a Jahn-Teller distortion by bending its π-backbone in order to facilitate spin-pairing between singly occupied molecular orbitals. This relationship between the excited-state dynamics of RC and its π-backbone geometry illustrates a potential strategy for developing π-conjugated radicals with new emission properties. Additionally, by comparing TDDFT and CIS (configuration interaction singles) excitations, we show that unrestricted TDDFT accurately reproduces experimental absorption spectra and provides an opportunity to examine the relaxed excited-state properties of large open-shell molecules like RC.
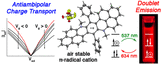
Open-Shell Effects on Optoelectronic Properties: Antiambipolar Charge Transport and Anti-Kasha Doublet Emission from a N-Substituted Bisphenalenyl
Journal ArticlePublisher:Journal of the American Chemical SocietyDate:2019Authors:Muhammad ImranCaleb M. WehrmannMark S. ChenDescription:By stabilizing unpaired spin in the ground state, open-shell π-conjugated molecules can achieve optoelectronic properties that are inaccessible to closed-shell compounds. Here, we report the synthesis and characterization of a N-substituted, bisphenalenyl π-radical cation [3(OTf)] that shows antiambipolar (anti-ohmic) charge transport and fluorescence via anti-Kasha doublet emission. 3(OTf) produces a red emission (634–659 nm) by radiative decay from β-LUMO to β-SOMO, based on density functional theory and configuration interaction singles calculations, and records one of the highest photostabilities (t1/2 = 9.5 × 104 s) among fluorescent radicals. Characterization of 3(OTf)-based field-effect transistors reveals that the observed electrical conductivity (σRT ≤ 1.3 × 10–2 S/cm) is enabled by hole and electron transport (μe/μh ≤ 5.70 × 10–5 cm2 V–1 s–1) that is most efficient in the absence of gating, which represents the first example of antiambipolarity in a molecular material.
Skills
- Organic Synthesis
- Single Crystal Growth
- Single Crystal XRD
- Physical Organic Chemistry
- Organic Semiconductors
- Fluorescence Spectroscopy
- Differential Scanning Calorimetry (DSC)
- Thermogravimetric Analysis (TGA)
- Electrochemistry
- Nuclear Magnetic Resonance (NMR)
- DFT
- Profilometer
- GC-MS
- MALDI-TOF
- ESI-MS
- High-Performance Liquid Chromatography (HPLC)
- Gel Permeable Chromatography
- Thin Films
- Spin Coating
- Column Chromatography
- Thin-Layer Chromatography (TLC)
Courses
Certifications
Teacher Development Certificate II
Issue date: Jun 2022,
Issued by: Center for Innovation in Teaching and Learning .
Description:Lehigh University
Teachings
Organic Spectroscopy
From: 2013, Until: 2016
Organization:Govt. Graduate College of Science FaisalabadField:Chemistry
Fundamentals of Organic Chemistry
From: 2011, Until: 2015
Organization:Govt. Graduate College of Science FaisalabadField:Chemistry
General Chemistry
From: 2009, Until: 2017
Organization:Govt. Graduate College of Science FaisalabadField:Chemistry
Image Gallery
Research Activities, Dissemination & Outreach
Poster presentation 10th Anniversary of Feng group at TU Dresden Dec. 2024
GRS 2021 at the University of New Mexico, Albuquerque, Division of Organic Chemistry, ACS.
Finalists Merck Chemist of Color Award 2021.
Group photo Chen lab 2020
Organic letters cover
Lorenzo Tesi giving an invited talk about about quantum qubits on solid surfaces.
Our synthetic sub-group social gathering at Bowling Arena Dresden Nov. 2023.
My daughters (Zoya and Alizeh) excited to visit my lab.
Future scientists curious about chemistry happening in fume hood.
Fulbright "Lab to Market" conference University of Utah May 2018.
Set up for generating dry CO2 gas from dry ice (frozen CO2) by passing through conc. H2SO4
Large scale (50g) reaction set up.
Air sensitive reactions under Argon baloons.
Preparative TLC separation.
Scientific Advisory Board (SAB) meeting Jan. 2025 at Max Planck Institute
Contact
Address
Department of Synthetic Materials and Functional Devices, Max Planck Institute of Microstructure Physics, Weinberg 2, 06120 Halle, Germany
-----------
Technische Universität Dresden
Department of Chemistry and Food Chemistry, Chair of Molecular Functional Materials, Walther-Hempel-Bau, Room 110, Mommsenstr. 4, 01069 Dresden, Germany